Which of the Following Has Eight Valence Electrons
The correct answer is D Elements with the most valence electrons are in group 18. Oxygen has atomic number of 8.

How Many Valence Electrons Does Copper Cu Have
Which of the following elements has eight valence electrons.

. Valence electrons in Oxygen O 6. Answer the questions below for an element that has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s1. It will be like Argon on the periodic table.
All of the noble gases except helium have 8 valence electrons. All of the above. Li ion configuration is 1s2 only two electrons in.
495 151 Views. Valence electrons in Silicon Si 4. From the electronic configuration of the oxygen we can see that there are six valence electrons present in its valence shell.
For B you can just tell by looking at the periodic table. C X e The atomic number of Xenon is 54 The electronic configuration of Xe is K r 4 d 10 5 s 2 5 p 6 It has 8 electrons in its valence shell and thus is a. Any element in group 18 has eight valence electrons except for helium which has a total of just two electrons.
Therefore atoms of chemical elements bond in order to attain the electronic configuration of a noble gas ie a full valence shell which comprises of eight 8 electrons. Which of the following has eight valence electrons. Valence electrons are the outermost shell electrons of an atom which it uses to form bonds with other atoms.
The atoms will be. There are only 5 valence electrons present in it. Which of the following has eight valence electrons.
B the energy given off when gaseous ions combine to form one mole of an ionic solid. Examples include neon Ne argon Ar and krypton Kr. It is a noble gas.
39 Votes Examples include hydrogen H lithium Li and sodium Na. How is helium different from the other elements in this group. The five elements noble gases neon Ne argon Ar kryptonKr xenon Xe and radon Rn have 8 valence electrons.
The valency remains the same along with the group and varies in the periodBut the group 18. Following the rule that each atom of carbon oxygen and nitrogen reacts to achieve a complete outer shell of eight valence electrons add unshared pairs of electrons as necessary to complete the valence shell of each atom in. The Group 6A parts have six valence electrons of their highest-energy orbitals ns2np4.
Valence electrons in Fluorine F 7. Valency is defined as the number of electrons present in the outermost shell. Your answer should be numerical and contain no words.
Valence electrons in Aluminum Al 3. Helium is in group 18 of the periodic table. Copper is a good conductor of electric current because it has eight or more electrons in its valence band.
It is also defined as the number of electrons available for the bond with the other element. Neon krypton argon and radon. E All of the above have eight valence electrons.
Na O CN3- A. A Ti4 B Kr C Cl- D Na E all of the above 2 Which of the following does not have eight valence electrons. 1 question Which of the following has eight valence electrons.
1 Which of the following has eight valence electrons. Examples include neon Ne argon Ar and krypton Kr. Cl has an extra electron because of the.
However the chemical element Hydrogen is an exception to the octet rule because it is only able to hold a maximum of two 2 valence electrons in its outermost shell to become full. Hence there are eight electrons present in the outermost shell of argon. Which of the following atomsions does not have eight 8 valence electrons.
Lattice energy is ________. Valence electrons in Sodium Na 1. The energy required to convert a mole of ionic solid into its constituent ions in the gas phase.
Asked Mar 14 2019 in Trades Technology by AppleFanBoy. Anything that is on the far right hand side of the periodic table has eight valence electrons. Which energy change corresponds to the electron affinity of fluorine.
Also what are valence electrons used for by an element. Sulfide S2- selenide Se2- and so on. Helium atoms have 2 valence electrons while atoms of the other elements in the group all have 8 valence electrons.
Any element in group 18 has eight valence electrons except for helium which has a total of just two electrons. This is barely two electrons away from having a full octet of eight electrons so many of those parts kind anions having -2 expenses. O Rb O Xe O Cat Br All of the above have eight valence electrons.
Valence electrons in Phosphorus P 5. Valence electrons in Sulfur S. A the energy required to convert a mole of ionic solid into its constituent ions in the gas phase.
Atomic number of beryllium is 4 and its electronic configuration is. - i thought it was b but its not and its not D either. A r 3 d 10 4 s 2 4 p 6 Since R b has 8 electrons in its valence shell and resembles the configuration of a noble gas Krypton it has a complete octet.
In a properly constructed Lewis dot structure for How many PAIRS of electrons are there in N2. Electrons in the outermost shell is known as valence electrons. 119 rows 8.
Rubidium has an atomic number of 37 The electronic configuration of R b will be. Only 2 electrons are present in its outermost shell. Valence electrons in Magnesium Mg 2.
Atomic number of chromium is 24 with electronic configuration. Valence electrons in Neon Ne 8. A Ca B Rb C Xe D Br- E All of the above have eight valence electrons.
All of these. In an oxygen element each oxygen atom contains eight electrons in its valence shell. In a properly constructed Lewis dot structure for How many PAIRS of electrons are.

How Many Valence Electrons Does Argon Ar Have

Valence Electrons Characteristics And Determination Of Valence Electrons
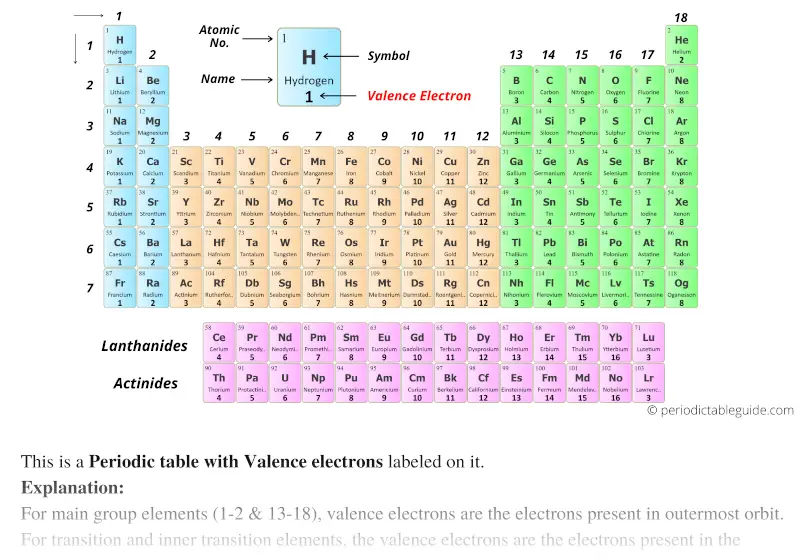
Periodic Table With Valence Electrons Labeled 7 Hd Images

How Many Valence Electrons Does Iron Fe Have
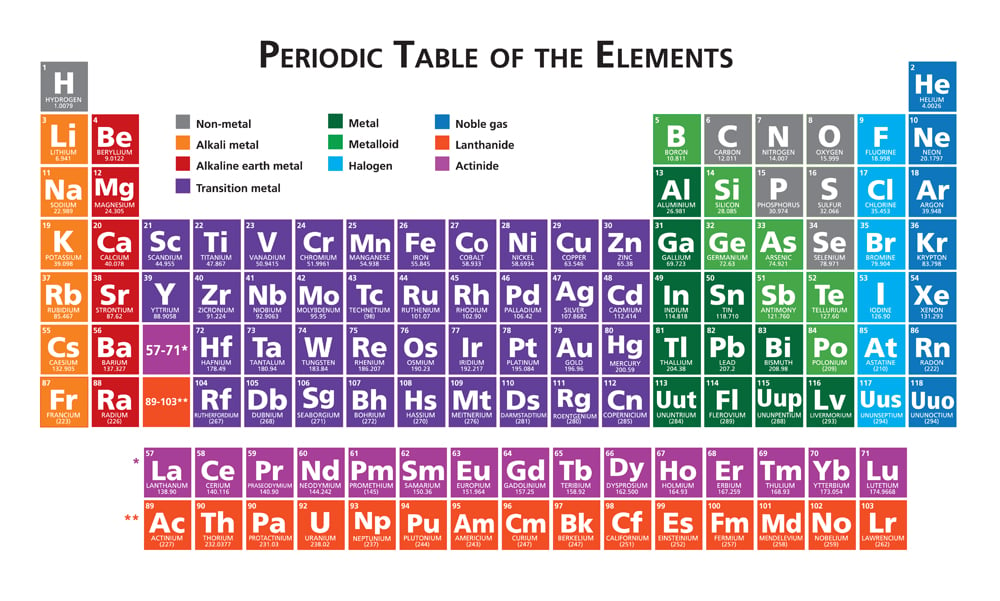
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable

Chemistry Classroom Chemistry Lessons Teaching Chemistry

What Are Valence Electrons Chemtalk

How To Find The Number Of Valence Electrons Using A Periodic Table Electrons Energy Level Chemistry

Why Are Atoms With 8 Valence Electrons So Stable Electron Configuration Electrons Covalent Bonding

Valence Electrons Tutorial Sophia Learning Electrons Education College Degree Program

How Many Valence Electrons Does Aluminum Al Have
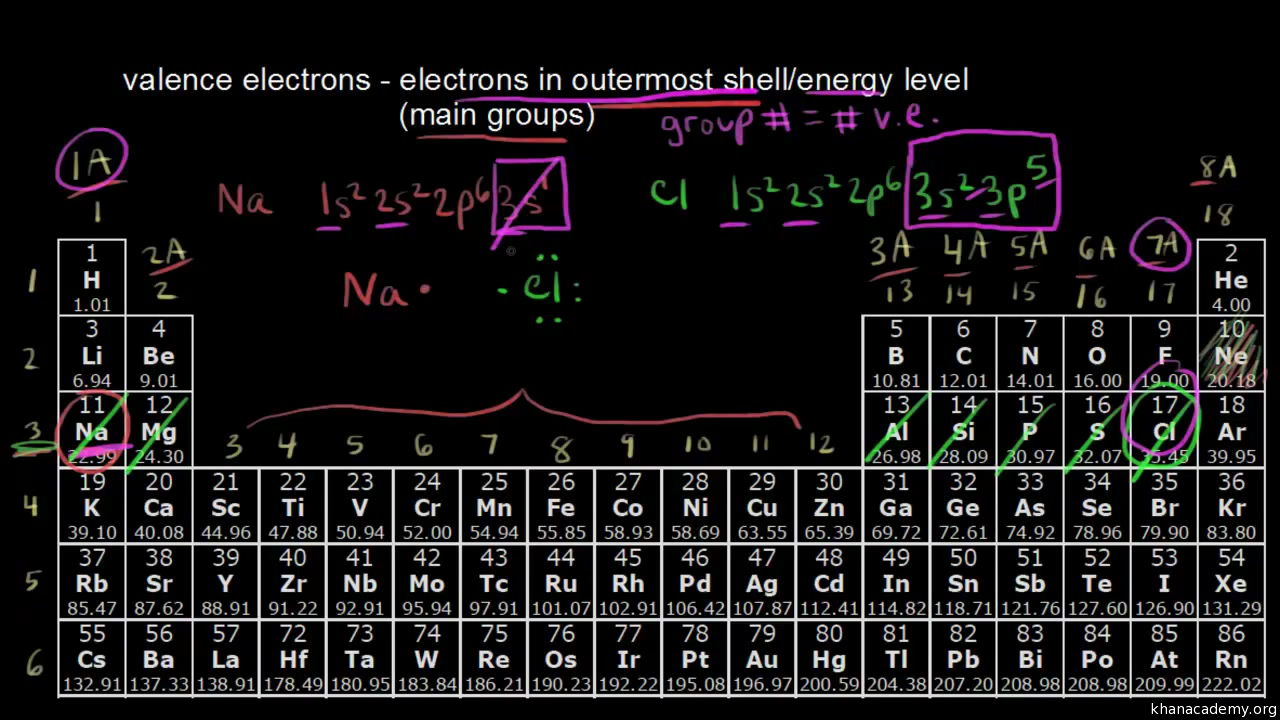
Counting Valence Electrons For Main Group Elements Video Khan Academy

Finding The Number Of Valence Electrons For An Element Youtube

How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Electron Configuration Business Plan Template



Comments
Post a Comment